What Are Three Examples Of Covalent Bonding
Therefore covalent solids have high melting points. Polar and Non- Polar Covalent.
Covalent Bond Images Stock Photos Vectors Shutterstock
The structure and bonding in a substance are modeled in different ways including dot and cross diagrams.

What are three examples of covalent bonding. The covalent bond classification CBC method is also referred to as the LXZ notation. The rearranging or breaking of covalent bonds requires large amounts of energy. Carbon and silicon.
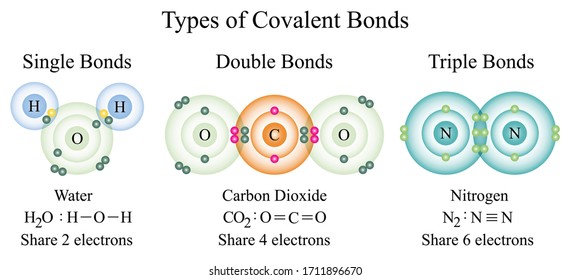
Bonds especially covalent bonds are often represented as lines between bonded atoms. There could be single double or triple covalent bonds between two elements depending on the number of electrons. Three dimensional configurations are best viewed with the aid of models.
Ionic bonding is the transferring of electrons while covalent bonding is the sharing of electrons--- classified into 2. This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners. Green in 1995 as a solution for the need to describe covalent compounds such as organometallic complexes in a way that is not prone to limitations resulting from the definition of oxidation state.
A covalent bond is a shared pair of electrons between atoms of two non-metal elements. Usually a single bond is a sigma bond. Electron pairs which participate in bonding are called bond pairs.
This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists Moore Stanitski and Jurs 393. Electron pairs which do not participate in bonding are called lone pairs. Chemical bonding is the sharing and transferring of electrons from the outermost shell.
CONSON JIMELLE - BSMT 1. Because there are no delocalized electrons covalent solids do not conduct electricity. Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds.
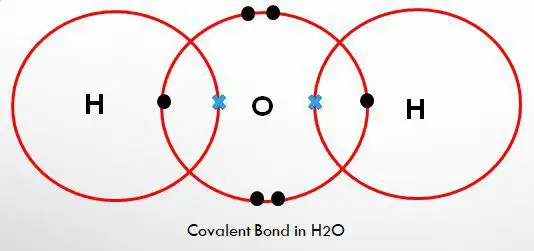
This pictures shows examples of chemical bonding using Lewis dot notationHydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen. It was published by M. Covalent or network solids are extended-lattice compounds in which each atom is covalently bonded to its nearest neighbors.
A covalent bond can also be a double bond or a triple bond. Covalent bond is formed between two non-metals by sharing of electrons. The three dimensional shape or configuration of a molecule is an important characteristic.
A single bond is weaker than either a double bond or a triple bond. Nitrogen forms three single covalent bonds to hydrogen atoms. This lesson enabled us to know more about the different chemical bonding.
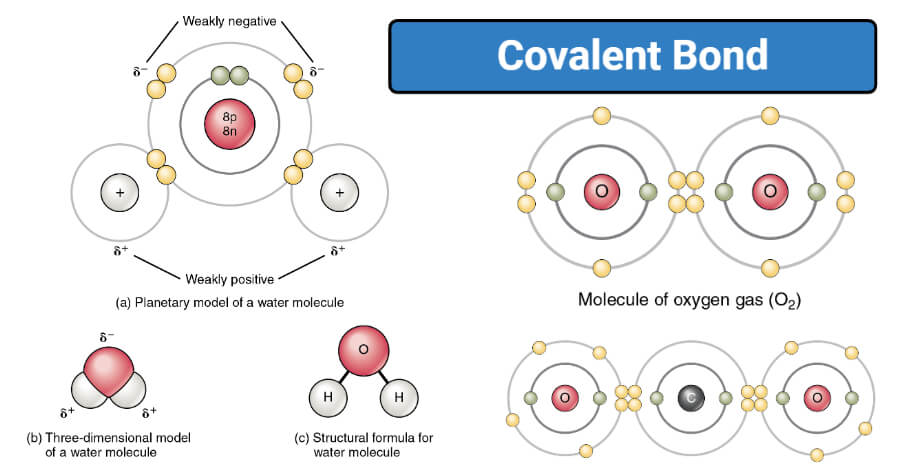
The bonding electrons in polar covalent bonds are not shared equally and a bond moment results. However a molecule may be polar or nonpolar depending on its geometry. For example tetrachloro-methane carbon tetrachloride CCl 4 has polar CCl bonds but the tetrahedral arrangement of the four bonds about the central carbon atom causes the individual bond moments to cancel.
Instead of simply assigning a charge to an atom in the molecule ie.
Ces Information Guide Materials Science Engineering

Covalent Bond Definition Types And Examples
Covalent Bond Definition Types Polar And Non Polar Covalent Bond Explained

Covalent Bond Definition Properties Examples Facts Britannica

Single Covalent Bond Definition And Examples
Covalent Bond Definition Properties Types Formation Examples

Covalent Bond Definition Types And Examples
Difference Between Covalent Metallic And Ionic Bonds With Comparison Chart And Similarities Bio Difference
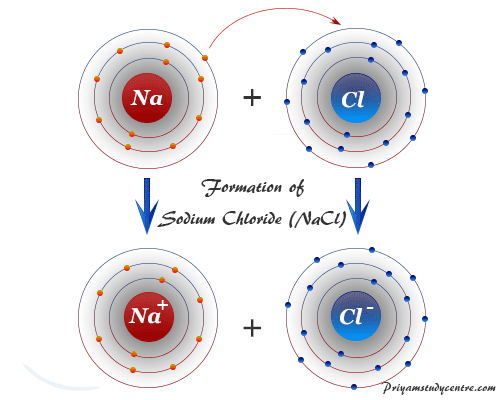
Covalent Bond Types Definition Properties Examples

Covalent Bonds Biology For Majors I

Covalent Bonding Biology Definition Role Expii

When A Metalloid Not A Metal Bonds With A Nonmetal Is It Ionic Or Covalent Quora

Covalent Bond Biology Dictionary
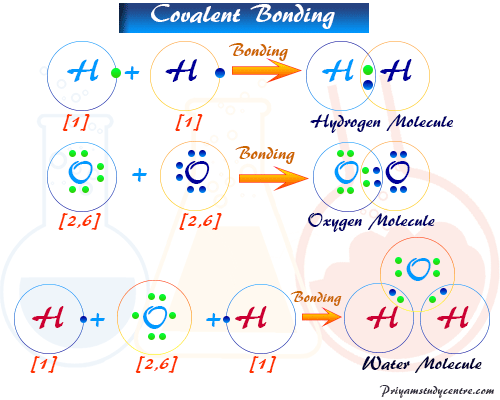
Covalent Bond Types Definition Properties Examples
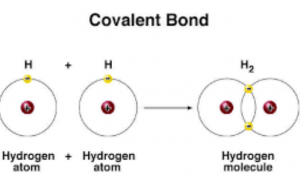
What Are The Types Of Covalent Bonds Science Online

Covalent Bond Definition Types And Examples

Notes 5 3 Covalent Bonds Covalent Bond A Force That Bonds Two Atoms Together By A Sharing Of Electrons Each Pair Of Shared Electrons Creates Ppt Download
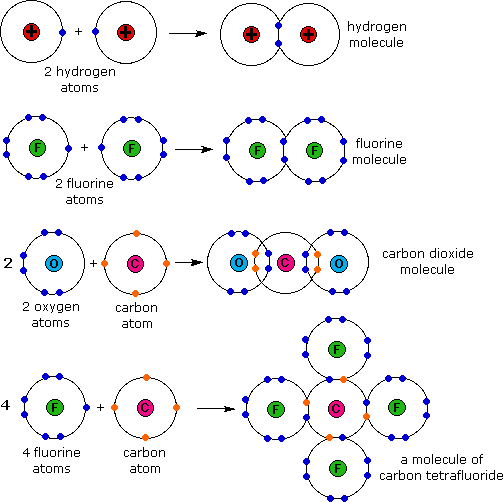
As Studypeach Covalent Bonds Sharing Is Caring
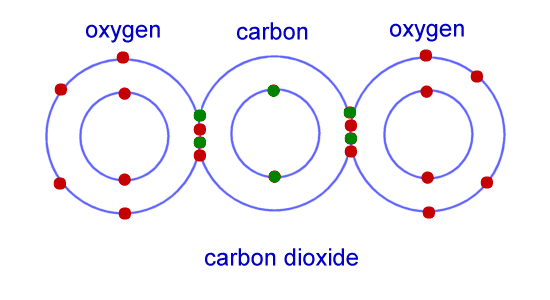
Chemistry For Kids Chemical Bonding